Selection of Broad-spectrum Anti-tumor Target: Targeting Methionine Metabolic Pathway for Anti-tumor (II)
Selection of Broad-spectrum Anti-tumor Target: Targeting Methionine Metabolic Pathway for Anti-tumor (II)
methionine (Met) is an essential amino acid in mammals, a sulfur-containing protein amino acid, and an essential amino acid for the initiation of protein synthesis [1].
1. The role of methionine in the body
In cells, methionine (Met) is mainly metabolized by the methionine cycle. Methionine cycle can produce methyl donor S-adenosylmethionine (SAM) involved in cell methylation reaction, has a wide range of physiological functions [2]. Methionine uptake and metabolism are involved in a variety of cellular functions, including methylation reactions, redox maintenance, polyamine synthesis, and coupling to folate metabolism, thereby coordinating nucleotide and redox states (Figure 1-1). It is the main methyl donor in cells and can methylate a variety of macromolecules, such as DNA, RNA, proteins, and lipids [3]. Methionine is synthesized de novo in most prokaryotes, despite differences in the enzymes that drive biological-level association [4]. Vertebrates cannot autonomously synthesize methionine in the body, and they must obtain this amino acid from external sources, such as diet and intestinal flora [5]. Obtaining methionine from the diet has a great influence on the metabolism of intracellular methionine.

Figure 1-1 The role of methionine in biological processes [6]
2, methionine metabolism
methionine is a special essential amino acid, since its long-term deprivation does not inevitably damage the life of the organism. However, many malignant cell lines, such as lung cancer, breast cancer, colon cancer, kidney cancer, etc., do not exhibit a complete and functionally capable mechanism for methionine synthesis. Because tumor cells divide faster than normal cells, they have a very high demand for methionine, which makes methionine the best choice for acid depletion therapy. Hoffman and Erbe[7] proved that although tumor cells can synthesize methionine endogenously, it is still insufficient to maintain the growth of tumor cells.
In addition to the exogenous supply, the cell can obtain methionine through a recycling pathway. This anaplastic pathway is dependent on methylthioadenosine phosphorylase and methionine synthase and is closely related to polyamine metabolism. The metabolism of methionine produces SAM to participate in the synthesis of polyamines, and the by-product of polyamine synthesis, methylthioadenosine, can be regenerated into methionine under the catalysis of methylthioadenosine phosphorylase, which also produces adenine.
Methionine depletion in methionine-dependent tumor cells can lead to cell cycle arrest in the late S/G2 phase, and cells arrested in the late S/G2 phase are prone to spontaneous death and are more sensitive to chemotherapeutic drugs [8]. This tumor-specific metabolic defect selectively kills tumor cells in co-culture with normal cells in combination with the chemotherapeutic agents doxorubicin and vincristine in vitro [9]. In addition, MX-1 human breast cancer cells grown in nude mice are highly sensitive to the combination of a methionine-deficient diet and cisplatin, but mice have some resistance to each alone [10]. A study by Machover et al., demonstrated synergistic cytotoxicity of recombinant methioninase with 5-fluorouracil and folinic acid in human leukemia cells [11].
Studies have shown that tumor initiating cells (TICs) have highly elevated methionine cycle activity and transmethylation rates driven by methionine adenosyltransferase (MAT2A). High methionine cycle activity results in methionine consumption far exceeding its regeneration, leading to exogenous methionine addiction. Therefore, the development of new anti-tumor therapies for abnormal methionine metabolism in tumor cells has great potential. So far, many anti-tumor drugs targeting the methionine metabolic pathway have entered the clinical stage.
Compared with normal tissues, tumor cells are widely dependent on exogenous methionine for survival, a phenomenon known as methionine dependence or Hoffmann effect [12]. Under conditions of methionine depletion, the proliferation of tumor cells is impaired, in part because of cell cycle arrest in many tumor cells, including lung adenocarcinoma, fibrosarcoma, and renal carcinoma. Methionine is metabolized by the methionine cycle and is involved in nucleotide biosynthesis, glutathione synthesis, and nucleotide/histone methyltransferase reactions. The essential amino acid methionine is important for the growth and metabolism of tumor cells. There is increasing evidence that methionine restriction inhibits tumor cell growth and may enhance the efficacy of chemotherapeutic drugs [13]. Dietary restriction of methionine in both prostate cancer cell xenograft models and metastatic tumor animal models resulted in tumor regression in mice. Clinical trials have shown that the combination of dietary restriction of methionine and chemotherapy in the treatment of gastric cancer patients is better than the use of chemotherapy alone. However, the method of dietary restriction of methionine content can only be implemented in the short term, with little effect; if implemented for a long time, it is easy to cause malnutrition. Since dietary restriction is often not accepted by patients, enzyme replacement therapy with methioninase may be more effective and better tolerated than dietary restriction. The specific consumption of methionine with methioninase has more potential for clinical application than dietary restriction of methionine alone.
3. The development status of methionine metabolism in the treatment of malignant tumors
According to the metabolic characteristics of methionine in tumors, the current methionine-related cancer treatment methods mainly focus on two directions: methionine depletion therapy and targeted therapy.
a. Methionine Depletion Therapy
, from the metabolic characteristics of methionine, the theoretical basis of methionine depletion therapy is mainly two points: first, methionine is an essential amino acid, so the restriction of methionine intake can reduce the level of methionine in the body. Secondly, tumor cells are dependent on exogenous methionine, and the lack of methionine can inhibit tumor growth and proliferation. In animal experiments, studies have found that methionine depletion therapy has a good inhibitory effect on the growth and metastasis of malignant tumors. In addition to its anti-tumor effect alone, methionine also has a synergistic effect with a variety of chemotherapeutic drugs such as 5-fluorouracil. In addition to limiting methionine intake, oral recombinant methioninase is another method of methionine depletion therapy, and has been studied in advanced prostate cancer, breast cancer, drug-resistant osteosarcoma, bladder cancer and other malignant tumors.
Methioninase, either alone or in combination with chemotherapy, is effective in inhibiting tumor growth in mice and rats. The study found that methioninase can effectively inhibit the growth of orthotopic xenograft tumor (PDX model) of tumor tissue from patients. Recombinant methioninase and chemotherapy regimens slowed the growth of Daoy, SWB77, and D-54 brain tumors in athymic mice. This was related to the depletion of methionine in the mouse plasma. The methionine concentration in the mouse plasma decreased to below 5mmol for several days after treatment. Methioninase depletion of methionine also caused extensive necrosis of tumor tissue. In 2017, Robert M Hoffman research group treated BRAF-V600E mutated melanoma orthotopic xenograft model with recombinant methioninase. The results showed that the tumor growth of the treatment group was inhibited, and the combination group of temozolomide and recombinant methioninase had better effect than the group using recombinant methioninase alone. The results showed that recombinant methioninase combined with first-line chemotherapy was very effective for melanoma and had certain clinical potential [14].
In 1996, RM Hoffman research group conducted a phase I trial for patients with advanced breast cancer, and confirmed that intravenous methioninase is safe and can consume methionine in serum without any signs of side effects [15]. In 1997, a phase I trial proved that recombinant methioninase is safe and effectively consumes methionine in serum for patients with advanced breast cancer, lung cancer, renal cancer and lymphoma, indicating the potential efficacy of methioninase in future clinical trials [16]. However, more than 20 years have passed since the initial phase I trial, and no further research has been conducted.
Methioninase is present in bacterial and fungal cells, but not in mammals. One of the main disadvantages of bacterial-derived methioninase is its poor stability in serum, and exogenous administration can easily cause immune response in human body [17]. Moreover, the half-life of methioninase in animals is relatively short, and one administration often fails to meet the therapeutic requirements, and multiple administrations increase the risk. The reduction of methioninase, whether by intravenous or intraperitoneal injection, represents a reduction in the level of methioninase in the whole body, and has a limited therapeutic effect on tumors. Therefore, the administration route using a vector to express the methioninase gene can be an effective solution.
B. Targeting methionine in vivo metabolic pathways
In the process of tumorigenesis, the gene expression regulation of methionine metabolic enzymes often changes. Restoring the normal function of these enzymes is expected to become another solution for the treatment of related tumors. At present, some new therapeutic strategies targeting methionine are being studied, namely targeting the methionine rescue pathway, targeting the polyamine metabolic pathway, and targeting the methionine cycle pathway. Such as PRMT5 and MAT2A, which play an important role in the methionine cycle; in the polyamine metabolic pathway, ornithine decarboxylase is the key enzyme in polyamine metabolism, its function is to control the rate of polyamine synthesis in cells, and has a direct regulatory effect on cell growth. The enzyme is used as an anti-tumor molecular target. Difluoromethyluric acid (DFMO) is an ornithine decarboxylase inhibitor, and studies have shown that its combined use with polyamine transport inhibitor AMXT-1501 may exert anti-tumor effects by preventing T cell immunosuppression [18]. However, there is no successful progress in clinical research in this field, and more relevant preclinical research may be needed to provide more accurate drug design direction.
Both of these two drug development ideas have certain preclinical efficacy, but also have their own advantages and disadvantages.
Table 1-1 Analysis of the advantages and disadvantages of methionine depletion treatment methods
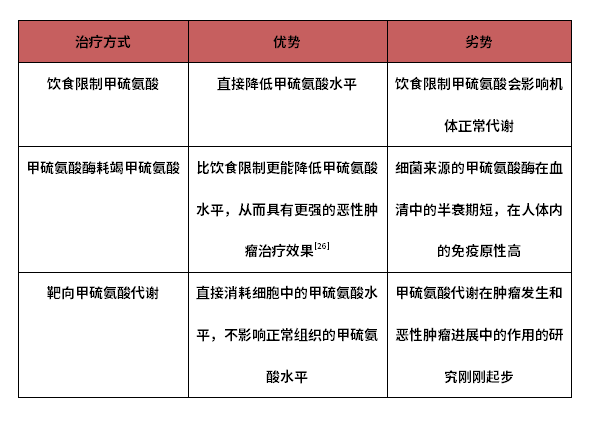
Note: The above main contents are taken from the research report of Yadu.
References
1. Kozak, M., Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev, 1983. 47(1): p. 1-45.
2. Murima, P., J.D. McKinney, and K. Pethe, Targeting bacterial central metabolism for drug development. Chem Biol, 2014. 21(11): p. 1423-32.
3. Cantoni, G.L., Biological methylation: selected aspects. Annu Rev Biochem, 1975. 44: p. 435-51.
4. Kalan, E. B. and J. Ceithaml, Methionine biosynthesis in Escherichia coli. J Bacteriol, 1954. 68(3): p. 293-8.
5. Neis, E.P., C.H. Dejong, and S.S. Rensen, The role of microbial amino acid metabolism in host metabolism. Nutrients, 2015. 7(4): p. 2930-46.
6. Sanderson, S.M., et al., Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer, 2019. 19(11): p. 625-637.
7. Hoffman, R.M. and R.W. Erbe, High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc Natl Acad Sci U S A, 1976. 73(5): p. 1523-7.
8. Poirson-Bichat, F., et al., Methionine depletion enhances the antitumoral efficacy of cytotoxic agents in drug-resistant human tumor xenografts. Clin Cancer Res, 2000. 6(2): p. 643-53.
9. Stern, P.H. and R.M. Hoffman, Enhanced in vitro selective toxicity of chemotherapeutic agents for human cancer cells based on a metabolic defect. J Natl Cancer Inst, 1986. 76(4): p. 629-39.
10. Hoshiya, Y., et al., Human tumors are methionine dependent in vivo. Anticancer Res, 1995. 15(3): p. 717-8.
11. Machover, D., et al., Cytotoxic synergism of methioninase in combination with 5-fluorouracil and folinic acid. Biochem Pharmacol, 2001. 61(7): p. 867-76.
12. Kaiser, P., Methionine Dependence of Cancer. Biomolecules, 2020. 10(4).
13. Wanders, D., K. Hobson, and X. Ji, Methionine Restriction and Cancer Biology. Nutrients, 2020. 12(3).
14. Kawaguchi, K., et al., Recombinant methioninase (rMETase) is an effective therapeutic for BRAF-V600E-negative as well as -positive melanoma in patient-derived orthotopic xenograft (PDOX) mouse models. Oncotarget, 2018. 9(1): p. 915-923.
15. Tan, Y., et al., Serum methionine depletion without side effects by methioninase in metastatic breast cancer patients. Anticancer Res, 1996. 16(6C): p. 3937-42.
16. Tan, Y., et al., Recombinant methioninase infusion reduces the biochemical endpoint of serum methionine with minimal toxicity in high-stage cancer patients. Anticancer Res, 1997. 17(5B): p. 3857-60.
17. Fernandes, H.S., et al., Amino acid deprivation using enzymes as a targeted therapy for cancer and viral infections. Expert Opin Ther Pat, 2017. 27(3): p. 283-297.
18. Hayes, C.S., et al., Polyamine-blocking therapy reverses immunosuppression in the tumor microenvironment. Cancer Immunol Res, 2014. 2(3): p. 274-85.


