[Frontier Science Popularization] The Truth of "Starving Tumor" -- Targeting Amino Acid Metabolism to Regulate Anti-tumor (I)
[Frontier Science Popularization] The Truth of "Starving to Death of Tumors"-Targeting Amino Acid Metabolism to Regulate Anti-tumor (I)
tumors are currently the main cause of disease deaths in the world. According to statistics from the International Cancer Research Agency, there will be about 19.3 million new tumor cases and nearly 10 million tumor deaths worldwide in 2020. The global cancer burden is expected to reach 28.4 million cases in 2040, an increase of 47% over 2020 [1].
One of the signs of malignant tumor is the rapid proliferation of tumor cells. In order to adapt to the microenvironment of hypoxia and lack of nutrients, tumor cells achieve rapid growth and change their own energy metabolism, which is called metabolic reprogramming. Among them, glucose metabolism, amino acid metabolism and lipid metabolism are the main features of metabolic reprogramming, which are the key factors leading to tumor microenvironment immunosuppression and tumor immune escape. Normal differentiated cells mainly rely on mitochondrial oxidative phosphorylation for cell energy supply, while most tumor cells rely on aerobic glycolysis, this phenomenon is called "Warburg effect". Although Warburg effects have been recognized early, no good treatment has been developed. Decades later, people began to pay attention to the dependence of tumor cells on amino acids, and found that tumor cells usually rely on the supply of exogenous amino acids, and this phenomenon not only appears in the body's synthesis of essential amino acids, but also appears in several non-essential amino acids. Therefore, the concept of "starving tumor" has become the main idea of developing new methods of tumor treatment in recent years.
targeting amino acid metabolism is an attractive form of cancer therapy because it can reduce the need for genotoxic drugs to mitigate the associated toxicity of long-term therapy, selectively killing tumor cells by interfering with amino acids. The effect of amino acid depletion therapy can be further enhanced when combined with conventional therapy or targeting tumor cell-specific survival mechanisms. A number of therapeutic approaches targeting amino acid metabolism are currently under clinical evaluation. These approaches can be performed from different perspectives by targeting amino acid metabolism, namely inhibition of amino acid transporter protein function, inhibition of amino acid biosynthesis, and amino acid depletion therapy, respectively.
1. The important role of amino acids in malignant tumor cells
malignancies have several roles:(1) as alternative fuels (2) as biosynthetic materials (3) affecting tumor cell survival (4) regulating redox balance (5) as epigenetic and post-transcriptional regulators. Amino acids play a biosynthetic role mainly in cells with adequate nutrition, and limiting amino acid metabolism can selectively target highly proliferating tumor cells [2]. Non-essential amino acids can be synthesized de novo in normal cells, but this function is lost in malignant tumors, which ensures that normal cells are not affected by specific amino acid restrictions [3]. Compared with normal tissues, tumor cells have specific auxotrophy and higher nutritional requirements [4]. This makes amino acid restriction a viable therapeutic strategy. Therefore, people have focused their research on another strategy that can "starve the tumor"-by limiting the supply of amino acids to ultimately achieve anti-tumor effects [5].
Tumor cells and normal cells are different in amino acid metabolism and requirements, and among many amino acids, methionine is the only one that almost all malignant tumors rely on. Methionine is an essential amino acid in mammals. When methionine (methionine,Met) is deprived or replaced by its precursor homocysteine (Homocysteine,Hcy), tumor cells cannot proliferate, while the proliferation of normal cells is not affected by these conditions. More and more studies have shown that methionine restriction can inhibit tumor cell growth and may enhance the efficacy of chemotherapy drugs. Methionine restriction combined with chemotherapy or radiotherapy is a very promising treatment option in clinical application.
2. Principle of Targeting Tumor Amino Acid Metabolism to Treat Malignant Tumor
At present, the method of targeting amino acid metabolism can be developed from the inhibition of amino acid transporters, amino acid biosynthesis and amino acid depletion (Figure 1). Tumor cells meet the increased demand for amino acids by upregulating the expression of amino acid transporters. However, functional redundancy between amino acid transporters makes inhibition of amino acid transporters difficult to achieve therapeutic goals. Inhibition of enzymes involved in amino acid synthesis and synthetic pathways, such as phosphoglycerate dehydrogenase (PHGDH), or glutaminase (GLS), which when targeted to the biosynthetic pathway, results in a lack of in vivo efficacy of the drug due to metabolic compensation. Many drugs are not suitable in a clinical setting because tumor cells often increase the availability of the nucleotide salvage pathway, thereby mitigating the cytotoxic effects of drugs targeting the nucleotide synthesis pathway [6]. The goal of amino acid depletion therapy is to use heterologous enzymes or engineered human enzymes to break down and deplete amino acids and induce apoptosis in tumor cells. Among the three different therapies (Table 1), amino acid depletion therapy is a research hotspot that has attracted much attention in recent years and is the most potential research direction. And the depletion therapy against methionine has broad-spectrum antitumor properties [7].
Table 1 Analysis of advantages and disadvantages of amino acid metabolism types
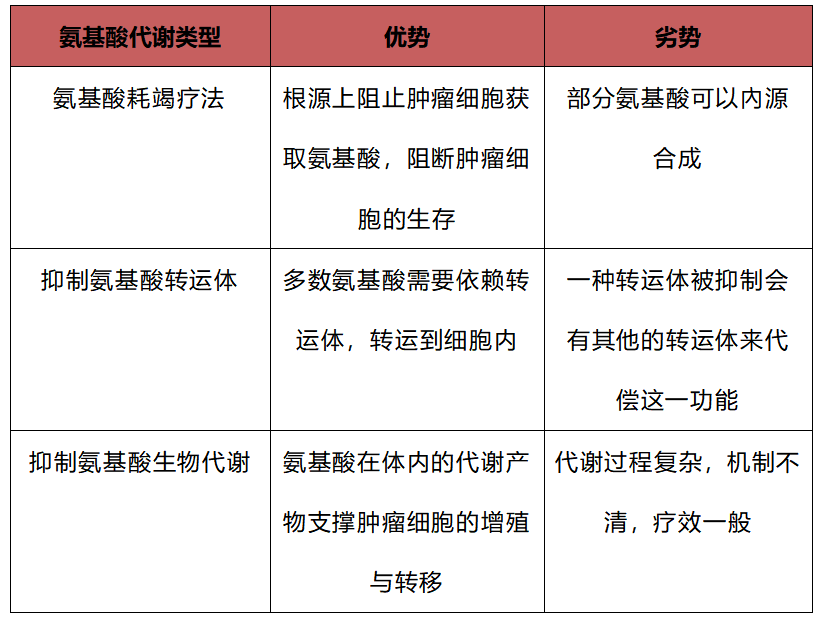

Figure 1 Malignant tumor therapeutic strategy targeting amino acid metabolism (tumor cells that rely on endogenous amino acid biosynthesis can be targeted by amino acid pathway inhibitors (purple). Tumor cells that depend on exogenous amino acid supply can be targeted by consuming this specific amino acid (blue). Normal cells have a much lower demand for amino acids, surviving either when amino acid biosynthesis is inhibited or when amino acids are depleted (gray).
ADI, arginine deiminase; EAA, essential amino acids; GLS, glutaminase; NEAA, non-essential amino acids, PHGDH, phosphoglycerate dehydrogenase.)
Note: The above main contents are taken from the research report of Yadu.
References
1. Sung, H., et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 2021. 71(3): p. 209-249.
2. Ganapathy-Kanniappan, S. and J.F. Geschwind, Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer, 2013. 12: p. 152.
3. Endicott, M., M. Jones, and J. Hull, Amino acid metabolism as a therapeutic target in cancer: a review. Amino Acids, 2021. 53(8): p. 1169-1179.
4. Fung, M.K.L. and G.C. Chan, Drug-induced amino acid deprivation as strategy for cancer therapy. J Hematol Oncol, 2017. 10(1): p. 144.
5. Pathria, G. and Z.A. Ronai, Harnessing the Co-vulnerabilities of Amino Acid-Restricted Cancers. Cell Metab, 2021. 33(1): p. 9-20.
6. Muhammad, N., H.M. Lee, and J. Kim, Oncology Therapeutics Targeting the Metabolism of Amino Acids. Cells, 2020. 9(8).
7. Butler, M., L.T. van der Meer, and F.N. van Leeuwen, Amino Acid Depletion Therapies: Starving Cancer Cells to Death. Trends Endocrinol Metab, 2021. 32(6): p. 367-381.


